- Systematic review update
- Open access
- Published:
Prevalence of thyroid dysfunction and associated factors among adult type 2 diabetes mellitus patients, 2000–2022: a systematic review and meta-analysis
Systematic Reviews volume 13, Article number: 119 (2024)
Abstract
Background
Thyroid dysfunction (TD) and type 2 diabetes mellitus (T2DM) frequently co-occur and have overlapping pathologies, and their risk increases with age. Thyroid dysfunction along with T2DM will worsen macro- and microvascular complications, morbidity, and mortality.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guideline was followed. The databases used were Embase, ScienceDirect, PubMed, and Google Scholar. The Joana Briggs Institute (JBI) scale was used to assess the quality of the included studies. The data was extracted by Microsoft Excel and analyzed through STATA version 14 software. The overall pooled prevalence of TD and its main components were estimated using the random-effects model. The consistency of studies was assessed by I2 test statistics. Pooled meta-logistic regression was used to present the pooled prevalence with a 95% confidence interval (CI). Besides, subgroup and sensitivity analyses were employed.
Result
Thirty-eight studies were included. The pooled prevalence of TD was 20.24% (95% CI: 17.85, 22.64). The pooled prevalence of subclinical hypothyroidism, hypothyroidism, subclinical hyperthyroidism, and hyperthyroidism was found to be 11.87% (95% CI: 6.90, 16.84), 7.75% (95% CI: 5.71, 9.79), 2.49% (95% CI: 0.73, 4.25), and 2.51% (95% CI: 1.89, 3.13), respectively. Subgroup analysis based on continent revealed a higher prevalence of TD in Asia and Africa. Factors like being female, HbA1c ≥ 7%, DM duration > 5 years, family history of TD, central obesity, smoking, the presence of retinopathy, and neuropathy were found associated with TD.
Conclusion
The current systematic review and meta-analysis showed that the TD’s pooled prevalence was relatively higher than the general population. Therefore, regular screening of TD should be done for T2DM patients.
Introduction
Thyroid dysfunction (TD), the most common endocrinal pathology next to diabetes mellitus (DM) [1], is a condition characterized by an increased or decreased production of thyroid hormones (TH) [2]. TDs occur as hypothyroidism (clinical or subclinical) or hyperthyroidism (clinical or subclinical) and are reflected in circulating levels of free triiodothyronine (FT3), free thyroxine (FT4), and TSH [3]. Type 2 diabetes mellitus (T2DM) is characterized by hyperglycemia as a result of insulin resistance and impaired pancreatic beta-cell activity [4, 5]. Obesity, a sedentary lifestyle, energy-dense foods, smoking, alcohol intake, and population aging are the key risk factors for T2DM [6].
Type 2 diabetes mellitus and TD often co-occur and have overlapping pathologies, and their risk increases with age. TDs are significantly more prevalent among T2DM patients [1]. TDs affect approximately 10 to 15% of the patients with diabetes, whereas in non-diabetes, the prevalence is approximately 6% [3]. The prevalence of TD in T2DM varies between studies, ranging from very low (5.5%) to very high (75%) [7]. Furthermore, studies have also recorded a higher prevalence of TD (31.4%) among females with T2DM [8]. Subclinical hypothyroidism is the most common type of TD among the diabetic population [9,10,11].
There is a complex relationship between TD and DM that has yet to be discovered. The pathophysiological link between T2DM and TD is thought to be the outcome of a complex interaction of biochemical, genetic, and hormonal abnormalities [12]. T2DM influences the TH in two sites, first at the level of hypothalamus by controlling TRH release and second at the peripheral tissues by impairing the conversion of T4 to T3 [13, 14]. The hypothalamus–pituitary–thyroid axis may be disrupted by experimentally induced diabetes, which lowers plasma TRH and TSH levels, lowering TH synthesis. [15]. In addition to this, anti-diabetics such as sulfonylureas and thiazolidinedione group drugs (e.g., pioglitazone) can negatively impact thyroid function [12].
Thyroid dysfunction can also cause T2DM. Both hypothyroidism and hyperthyroidism have been investigated to be associated with DM [1]. Hypothyroidism is associated with reduced glucose absorption from GIT, and it is accompanied by prolonged peripheral glucose accumulation, diminished hepatic glucose output, and reduced utilization of glucose, which were considered hallmarks of diabetes [16]. On the other hand, hyperthyroidism promotes hyperglycemia, and several theories have been proposed to explain this impact. In a thyrotoxic environment, the half-life of insulin is shortened, which is assumed to be related to the accelerated degradation of the active hormone and the release of inactive precursors [17]. In addition, hyperthyroidism is also hypothesized to boost glucose production through a variety of processes, including upregulation of gluconeogenesis as a result of increased lipolysis and lactate overproduction, as well as increased hepatic output due to increased expression of the GLUT2 glucose transporter [18].
The coexistence of TD in T2DM patients will worsen the macro-vascular and microvascular complications, morbidity, mortality, and quality of life [11]. Evidence indicates that subclinical hypothyroidism compromises both micro- and macrovascular function, increasing the risk of peripheral neuropathy, peripheral artery disease, and diabetic nephropathy [13, 19]. In addition to this, both subclinical hyperthyroidism and T2DM have been associated with an increase in cardiovascular disease risk and mortality [20]. Both TD and DM, especially uncontrolled diabetes, cause many health problems. Increased frequency of hypoglycemia in hypothyroidism and development of potentially life-threatening ketoacidosis in thyrotoxicosis are the most serious effects [21].
Detecting TD in T2DM patients would help clinicians provide the best treatment for metabolic problems, as TDs like hypothyroidism can make achieving a glycemic target and managing other comorbidities difficult [11]. Screening of TD, especially the subclinical dysfunction, in patients with DM is justified because most patients can be asymptomatic [22]. The strong link between diabetes and TD encouraged the American Diabetes Association to propose that people with diabetes must be checked periodically for TD [23].
There are different studies conducted to assess the prevalence and associated factors of TD among T2DM all over the world. Despite their results having a great disparity and inconsistent findings, moreover, there is no previous systematic review and meta-analysis that estimated the prevalence and associated factors of TD among T2DM. Therefore, the current systematic review and meta-analysis is designed to assess the pooled prevalence and associated factors of TD among T2DM patients.
Methods and materials
Eligibility criteria
Inclusion criteria
Studies on the prevalence and associated factors of TD among adult T2DM patients published in different peer-reviewed journals between 2000 and 2022 were included. All studies were original research published in English and contained the minimum information concerning sample size and status of TD, which helped to analyze a pooled estimate of the prevalence of TD and associated factors among adult T2DM patients. Besides, studies in which TD has been classified as hypothyroidism, hyperthyroidism, subclinical hypothyroidism, and subclinical hyperthyroidism using laboratory measurements of TSH, T4, and T3 were included.
Hypothyroidism is characterized by elevated serum TSH levels, a low serum FT4 level, and low FT3 [24, 25], whereas hyperthyroidism is characterized by elevated serum FT4 and FT3 and low levels of TSH levels [26]. Subclinical hyperthyroidism is characterized by decreased serum TSH concentration in association with a normal serum FT4 and FT3 concentrations [26]. Subclinical hypothyroidism is defined as an elevated serum TSH level associated with normal total or FT4 and FT3 levels [20]. Studies that used International Diabetes Federation (IDF) criteria for diagnosing T2DM were included. The IDF criteria state diagnostic criteria for diabetes which is maintained fasting plasma glucose ≥ 7.0 mmol/l (126 mg/dl) or 2–h plasma glucose ≥ 11.1 mmol/l (200 mg/dl) [27].
Exclusion criteria
Articles written in another language other than English were excluded. Studies conducted among type 1 DM patients, and diabetic neuropathy patients, were excluded. Studies from non-original papers (literature reviews, books) were also excluded. Irrelevant and duplicated papers were excluded. Articles which lacked necessary information such as age and year of study were also excluded. Studies that did not show the diagnostic criteria for both T2DM and TD were omitted. Furthermore, articles that did not provide information on the overall prevalence of TD were omitted.
Search strategy
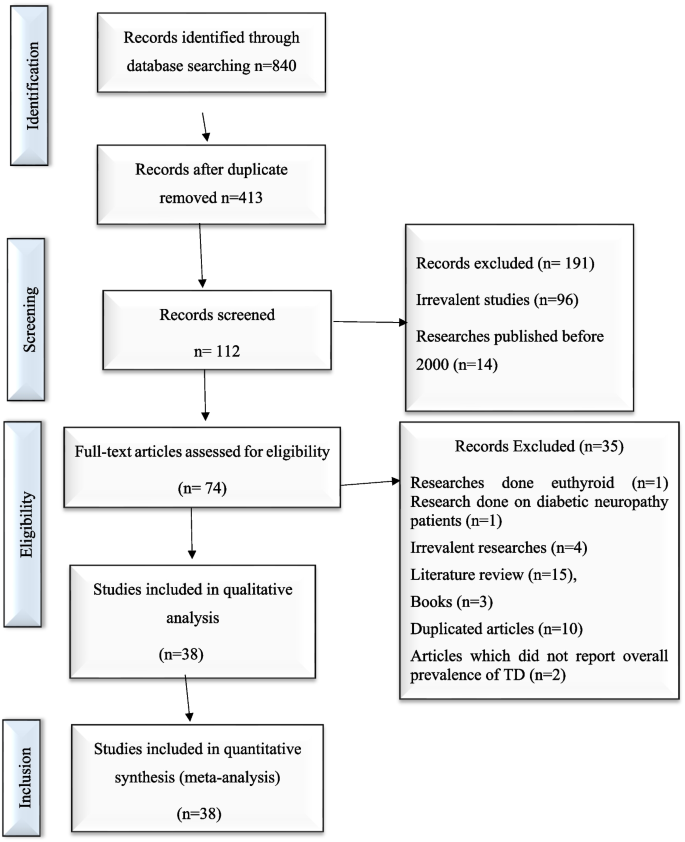
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guideline was used to report this systematic review and meta-analysis [28]. An electronic search was conducted to retrieve studies. Published articles of cross-sectional, case–control, cohort, prospective, case series, and retrospective studies were included. Embase, PubMed, Google Scholar, and ScienceDirect literature were the electronic databases used to identify studies conducted on the prevalence of TD and associated factors among T2DM patients published from 2000 to 2022. The search terms were used in agreement with the title/abstract using the arrangement of keywords that were used to select relevant studies. Figure 1 shows the flow chart used to describe the selection of studies.
Using Boolean operators like “OR” and “AND,” the search terms were utilized separately and in combination. An example of the search strategy used to retrieve relevant articles was as follows: (((((((prevalence[Title/Abstract]) AND (hypothyroidism[Title/Abstract])) AND (hyperthyroidism [Title/Abstract])) AND (thyroid disorders[Title/Abstract])) OR (thyroid dysfunction [Title/Abstract])) AND (adult[Title/Abstract])) AND (type 2 diabetes mellitus[Title/Abstract])) OR (insulin resistant diabetes[Title/Abstract]). Duplicated data were excluded. The software EndNote version X8 (Thomson Reuters, New York, NY, USA) was used to manage references and remove duplicated references.
Search method and quality assessment
An electronic search was conducted in Embase, PubMed, Google Scholar, and ScienceDirect literature using the keywords to include articles that were published from 2000 to 2022. Then, searched articles were screened by the title and abstract to consider the articles in the full-text review. Following the exclusion of duplicates, the abstracts and titles of 413 papers were screened for eligibility criteria, and 38 were chosen for full-text evaluation.
This systematic review and meta-analysis is based on original research articles. For maintaining the quality of the review, all duplications were checked thoroughly. The abstracts of these articles were checked deeply for the analysis and purification. A careful evaluation of each research paper was carried out at later stage.
The quality of the studies was assessed using the Joana Briggs Institute (JBI) standardized critical appraisal instrument for prevalence studies scale. The following items were used to appraise the included studies: (Q1) Was the sample frame appropriate to address the target population?, (Q2): Were study participants sampled in an appropriate way?, (Q3): Was the sample size adequate?, (Q4): Were the study subjects and the setting described in detail?, (Q5): Was the data analysis conducted with sufficient coverage of the identified sample?, (Q6): Were valid methods used for the identification of the condition?, (Q7): Was the condition measured in a standard, reliable way for all participants?, (Q8): Was there appropriate statistical analysis?, and (Q9): Was the response rate adequate, and if not, was the low response rate managed appropriately? Table 1 shows the methodological quality assessment of included studies using the Joana Briggs Institute (JBI) standardized critical appraisal instrument for prevalence studies scale.
Data extraction
An established data extraction tool, Microsoft Excel 2013 spreadsheet, was used for the data extraction. Three authors (R. H., A. W., and S. A.) independently conducted a search in Embase, PubMed, Google Scholar, and ScienceDirect databases. This tool extracted information such as the author’s name, publication year, study design, sample size, prevalence of TD, prevalence of subgroups of TD, and the laboratory diagnostic method used to diagnose TD, and T2DM were all extracted using this tool. PRISMA guideline was strictly followed when conducting this review.
Data processing and analysis
Data was entered and analyzed using STATA version 14 after extracting the data from all eligible studies. Overall, pooled prevalence of TD and its main components were estimated using the random-effects model. In the meta-analysis, to assess the consistency of studies, I2 test statistics was used. This test examines the hypothesis of all the included studies is evaluated for the same effect. Consequently, since there was heterogeneity between the original studies (I2 = 93.5%, p < 0.001), a random-effect model was needed. The presence of publication bias was evaluated by using funnel plot test. Besides, study bias was evaluated using Egger’s test. Moreover, in this study, forest plots were used to estimate pooled effect size and effect of each study with their confidence interval (CI) to provide a visual image of the data. Pooled meta-logistic regression was used to present the pooled prevalence with a 95% confidence interval. Besides, subgroup and sensitivity analyses were employed.
Results
Characteristics of the included studies
A total of 840 potential articles were identified through the systematic literature search. After removal of duplicates, 413 articles were screened by title and abstract, and 74 were found to be eligible for full-text assessment. Of these full-text-screened articles, 38 (including 19,803 study participants) were found to be eligible for meta-analysis. Table 2 shows the general characteristics and outcomes of included studies.
The studies that are included were done all over the globe and published between 2000 and 2022. The meta-analysis included 38 studies that revealed the prevalence and contributing factors of TD among T2DM patients. Among all the papers, 6 of them were from Africa [3, 31, 35, 43, 45, 51], 28 were from Asia [3, 7,8,9, 11, 14, 16, 23, 24, 29, 32,33,34, 37,38,39,40,41,42, 44, 46, 47, 49, 50, 52,53,54,55,56,57,58], 1 was from Australia [48], 2 were from Europe [10, 30], and 1 was from South America [22]. Regarding the study design, 9 were case–control, 1 case series, 3 were cohort, 21 were cross-sectional, 1 prospective, and 3 retrospective studies. The minimum sample size was 40 participants in a case–control study conducted in India [55], while the highest sample size was 2219 participants, in a cohort study conducted in India [49].
The Joana JBI standardized critical appraisal instrument for prevalence studies indicated that none of the included studies was of poor quality. After quality assessment, the 38 studies were subjected to meta-analysis. Table 2 presents the characteristics and outcomes of the reviewed studies. The prevalence of TD was estimated based on measurement of blood levels of TSH, FT3, and FT4 among the T2DM patients from all over the world.
Prevalence of TD among adult T2DM patients
Thirty-eight published studies were included in this systematic review and meta-analysis, and all of these studies were used to estimate the pooled prevalence of TD among T2DM patients.
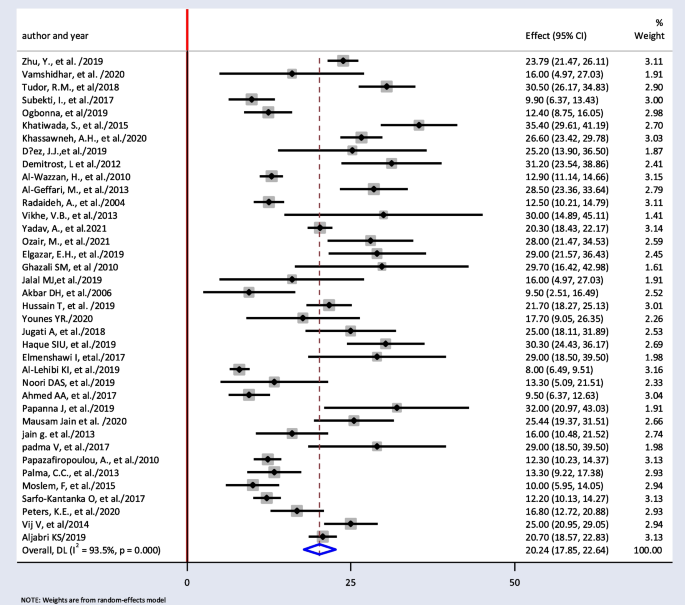
The minimum prevalence of TD was 8% from a retrospective study done in India [52], and the maximum prevalence of TD was found to be 35.4% in Nepal [32]. The I2 test result showed high heterogeneity (I2 = 93.5%, = 0.000). The pooled prevalence of TD among T2DM was found to be 20.24% (95% CI: 17.85, 22.64) using random-effect model (Fig. 2).
Prevalence of types of TDs and subgroup analysis
Thirty-four papers were used to estimate the pooled prevalence of subgroups of TD. The pooled prevalence of subclinical hypothyroidism, hypothyroidism, subclinical hyperthyroidism, and hyperthyroidism were found to be 11.87% (95% CI: 6.90, 16.84), 7.75% (95% CI: 5.71, 9.79), 2.49% (95% CI: 0.73, 4.25), and 2.51% (95% CI: 1.89, 3.13), respectively.
Subgroup analysis based on the study design showed that the weighted pooled prevalence of TD was 18.97% (95% CI: 15.93, 22.01), 22.17% (95% CI: 16.41, 27.92), 21.32% (95% CI: 14.37, 28.27), 22.33% (95% CI: 4.34, 40.32), 21.70% (95% CI: 18.27, 25.13), and 25% (95% CI: 18.11, 31.89) among the cross-sectional, cohort, case control, retrospective, prospective, and case series respectively. Table 3 shows the summary of the subgroup analysis of studies.
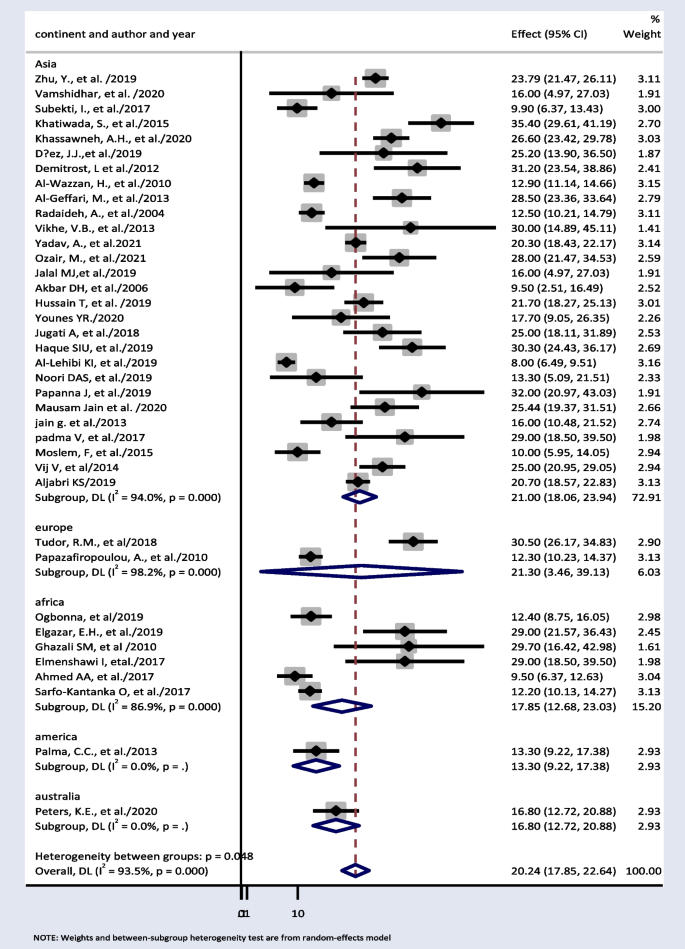
Thirty-eight papers were used to estimate subgroup analysis based on continent. The result showed weighted pooled prevalence of TD was 21% (95% CI: 18.06, 23.94), 21.30% (95% CI: 3.46, 39.13), and 17.85% (95% CI: 12.68, 23.03) in Asia, Europe, and Africa, respectively (Fig. 3).
Factors associated with TD among T2DM patients
In this meta-analysis, seven studies were included to examine the factors associated with TD among T2DM [30,31,32, 39, 44, 52, 59]. Being female [23, 30,31,32, 39, 44, 59], central obesity [31], HbA1c ≥ 7% [31, 44], > 5-year duration of DM [31, 44, 59], educational level [59], diabetic neuropathy and retinopathy [31, 59], family history of TD [23, 32], and smoking [32, 39, 44] were found to be associated with T2DM. Tables 4 and 5 shows summary statistics of the risk factors.
Sensitivity test
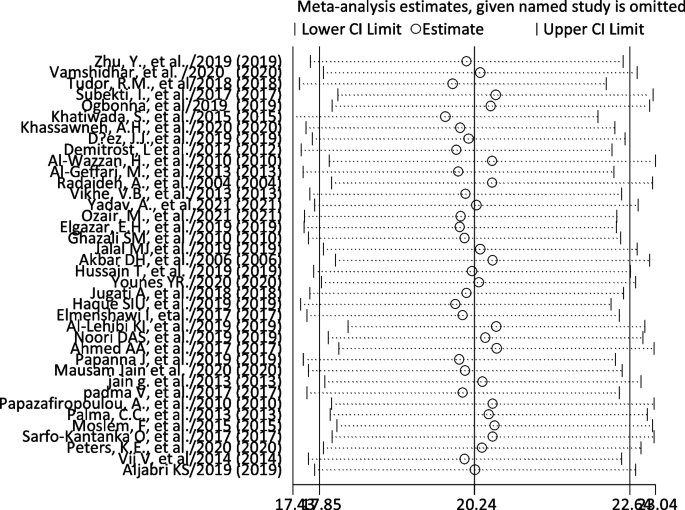
We did the sensitivity analysis of the prevalence of TD among T2DM by applying a random-effects model (Table 6). The analysis was done to evaluate the effect of each study on the pooled estimated prevalence of TD by excluding each study step by step. The result showed that excluded studies did not show a significant difference in the prevalence of TD among T2DM (Fig. 4 and Table 6).
Publication bias
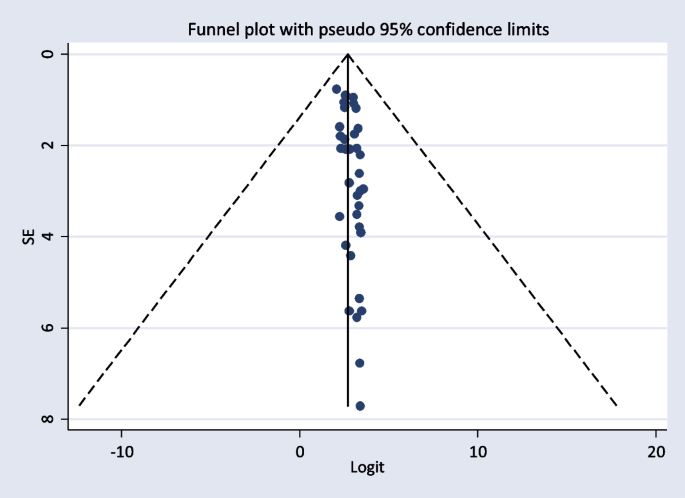
The included studies were assessed for potential publication bias visually by funnel plot. The funnel plot was asymmetrical which indicate the presence of publication bias (Figs. 4 and 5). Besides, the result of Egger’s test indicated there was publication bias, P-value < 0.05. The P-value was found to be 0.019 (Table 7).
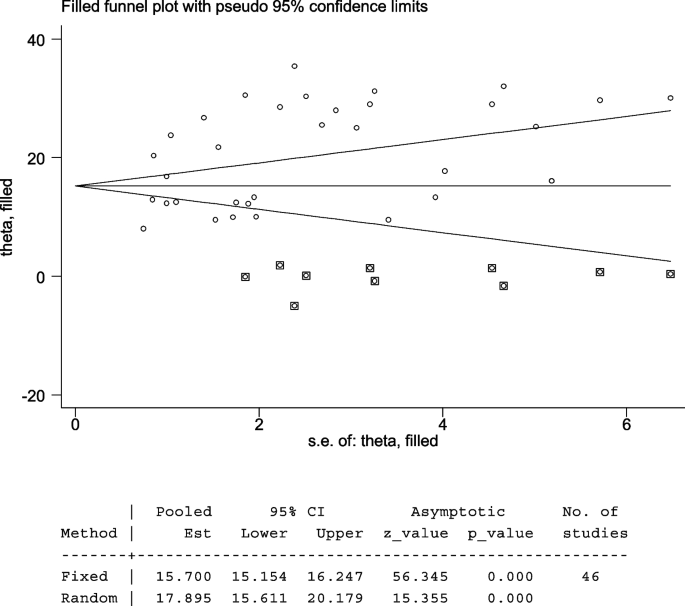
The Egger’s test indicated that the unpublished findings might have shown a lower magnitude of TD. Adjusting the findings using the trim-and-fill method would provide a bias-adjusted effect estimate. Therefore, to do so, a trim-and-fill method analysis was conducted. A bias-adjusted effect estimate of TD showed 17.89 (15.611, 20.179), assuming there are missing studies (Fig. 6).
Meta-regression
Meta-regression was performed to determine the source of heterogeneity by considering sample size and year of publication as a covariate. There was no significant relationship between the year of publication and the prevalence of TD. In addition to this, meta-regression was also conducted that explains the linear prediction of the prevalence of TD and function of sample size. Similarly, there was no significant relationship between the sample size and the prevalence of TD (Table 8).
Discussion
The pooled prevalence of TD in this systematic review and meta-analysis was found to be 20.24% (95% CI: 17.85, 22.64). Funnel plots and Egger’s tests showed there was publication bias among included studies. Trim-and-fill method was used to correct the results. A bias-adjusted effect estimate of TD showed 17.89 (15.611, 20.179), assuming there are missing studies. This result was higher than the 11.7% seen in a Colorado TD survey of 25,862 people who attended a state health fair [12]. It was also higher than the National Health and Nutrition Examination Survey (NHANES III Study), a survey of 17,353 subjects (5.9%) [60]. Type 2 diabetic patients have a higher prevalence of TD than non-diabetics; T2DM lowers TSH levels and impairs the conversion of T4 to T3 in the peripheral tissues. Poorly managed T2DM can lead to insulin resistance and hyperinsulinemia. This, in turn, promotes the growth of thyroid tissue and increases the formation of nodules and the size of goiters. In addition, while metformin can be beneficial in both T2DM and TD patients, there are some other antidiabetic drugs like sulfonylureas, and thiazolidinedione group drugs like pioglitazone can negatively impact thyroid function [12].
The most common type of TD seen in this systematic review and meta-analysis was subclinical hypothyroidism (11.87%, 95% CI: 6.90, 16.84). This result was in line with a systematic review and meta-analysis done globally (12% (95% CI: 10%, 14%) [61] and higher than results reported in general population (4–9%) [1]. More than half of TDs reported are undiagnosed or subclinical because symptoms of TD are easily mistaken for depression, menopause, or obesity [62]. The presence of subclinical hypothyroidism may increase cardiovascular risk by aggravating dyslipidemia, insulin resistance, obesity, and vascular endothelial dysfunction [1, 63].
Hypothyroidism was the second most common form of TD found in this systematic review and meta-analysis (7.75% (95% CI: 5.71, 9.79). This finding was also higher than that of the NHANES III Study (4.6%) [1]. Overall hypothyroidism is the most common type of TD among T2DM patients. Worldwide, environmental iodine deficiency is the most common cause of hypothyroidism [64]. Globally, more than 1.9 billion individuals have inadequate iodine nutrition Despite the implementation of iodine supplementation programs (e.g., salt iodization), iodine intake remains suboptimal in large parts of the world [64, 65].
In this review, the pooled prevalence of hyperthyroidism was 2.51% (95% CI: 1.89, 3.13). This was similar to the community-based study done in Wickham among 2779 participants (2%) [66].
Subgroup analysis based on continent showed 21% (95% CI: 18.06, 23.94) and 17.85% (95% CI: 12.68, 23.03) pooled prevalence of TD in Asia and Africa respectively. TDs have been documented in more than 110 countries, the most of which are in Africa, Asia, and Latin America [67]. In comparison with other continents, this result is high. This is because, in the developed world, the frequency of undiagnosed TD is anticipated to be declining as a result of extensive thyroid function testing and low treatment initiation thresholds. However, in continents such as Africa and Asia, this is challenge [68]. Iodine deficiency is a major public health problem throughout Africa and is the commonest cause of TDs in this continent [69]. At least 350 million Africans are at risk of iodine deficiency. A total of 25% of the global burden of iodine deficiency occurs in Africa [70].
Seven studies were included to examine the factors associated with TD among T2DM [30,31,32, 39, 44, 52, 59], and different factors were found associated with TD. Among them, sex was found to be the prominent determinant of TD. All of the studies indicated a statistically significant association between sex and TD that shows a higher risk of TD with being female. In a cross-sectional research of 411 T2DM patients in Saudi Arabia, it was discovered that being female has 1.95 higher odds of having TD as compared with males (OR = 1.95, 95% CI: 1.36–2.78, p = 0.0001) [23]. Female gender was also a risk factor for TD, according to a study conducted in Greece among 1092 T2DM patients (OR = 0.222, 95% CI = 0.141–0.352, p = 0.001) [30]. These results were also similar to the study conducted in Nepal and Kuwait (RR = 1.44, 95% CI = 1.09–1.91, p = 0.01) and (OR = 1.7, 95% CI: 1.2–2.9, p = < 0.001) respectively [32, 39].
In a research done in Nigeria among 354 T2DM patients, it was found females who had T2DM were 3.8 times more likely to develop TD than their male counterparts (OR = 3.8, 95% CI = 1.7–8.4, p = 0.002) [31]. Similar result was found in a case–control study conducted in Ethiopia. Being female had 2.5 times the odds of having TD than their male counterparts 2.5 (OR = 2.5, 95% CI = 1.15–5.67, p = 0.022) [59].
The prevalence of TD in diabetic patients is influenced by female gender in which T2DM patients who are female are more likely to develop TD. This in because sex hormones and the skewed inactivation of the X chromosome are suspected to be triggers for hypothyroidism and hyperthyroidism [71]. Another factor contributing to the high prevalence of TD in women is the interaction between TH and hormones that change during the menstrual cycle [72].
Smoking was also found associated with TD among T2DM patients [32, 39]. In the study conducted in Kuwait among 204 T2DM patients, ex-smokers and current smoker patients were more liable for TD (OR = 18.1, 95% CI: 10.1–32.5) and (OR = 7.8, 95% CI: 3.5–17.7) respectively [39]. Similar finding was also found in the study done in Nepal. Smokers had 2.32 higher odds of having TD (OR = 2.32, 95% CI: 1.85–2.91) [32].
The reason behind this is that cigarette smoke contains cyanide which is converted to thiocyanate, which disrupts iodine uptake and blocks the production of THs [73]. Many other components of cigarettes also have antithyroid effects, such as decreasing T3 receptor binding or post-receptor activities in the liver, muscle, or both. According to reports, smoking/nicotine causes an unnaturally high metabolism, masking the fatigue/lethargy associated with hypothyroidism. When the smoker quits, this masking is removed, and the full effects of hypothyroidism on the metabolism and thyroid are felt. And, for smokers with undiagnosed TD, without proper TH treatment, smoking cessation seems to double weight gain whammy, as they lose the appetite suppressant, metabolism-upping effects of nicotine, and experience the full effects of the hypothyroidism [39].
Besides, among the seven papers used to assess associated factors, two of them reported that TD is associated with HbA1c ≥ 7% [31, 44]. A study conducted in Nigeria found that T2DM patients with ≥ 7% HbA1c were 4.3 times more likely to develop TD than their counterparts with good glycemic control (HbA1c < 7%) (OR = 4.3, 95% CI = 2.1–8.9, p = 0.025) [31]. A case–control study conducted in Jordan was also in line with this study. It was found that patients who had HBA1c ≥ 7% were found to have 2.55 higher odds of having TD when compared with patients who have HBA1c ≤ 7% (OR = 2.55, 95% CI = 1.45–4.43, p = 0.001) [44]. The association of hyperglycemia with TD may be due to the adverse effects of chronic hyperglycemia on the hypothalamic-pituitary axis where it blunts or abolishes the nocturnal TSH peak [59].
It was also found central obesity (abnormal waist circumference) was significantly associated with TD in a case–control study done in Nigeria (OR = 2.5, 95% CI = 1.5–5.2, p = 0.001) [31]. Leptin is known to be an important neuroendocrine regulator of the hypothalamo-pituitary-thyroid axis by regulating TRH gene expression in the paraventricular nucleus. Iodine deficiency, autoimmune thyroiditis, and mutations in the TSH receptor genes are some of the other hypotheses put forward to explain the association between increasing TSH, obesity, and subclinical hypothyroidism in some populations [31].
Duration of diabetes was found to be associated with TD in two of the studies [23, 31]. In a research done in Nigeria among 354 T2DM patients, DM duration > 5 years (OR = 3.3, p = 0.012) was a risk factor for TD [31]. A cross-sectional study conducted in Saudi Arabia diabetes also reported that duration of more than 10 years has been shown to be an important risk factor (OR = 1.66, 95% CI: 1.06–2.61) [23]. This could indicate that the duration of diabetes mellitus (DM) is a risk factor for the development of TD, as persistent hyperglycemia inhibits the peripheral deiodination of T4 to T3, resulting in TD [31].
Educational level was also found associated with TD. It was found T2DM patients who attend primary school had 1.5 higher odds of having TD (OR = 1.5, 95% CI: 1.03–1.67). On the other hand, T2DM patients who have secondary education and post-secondary education had less likely to have TD (OR = 0.11, 95% CI = 0.06–0.48, p = 0.02) and (OR = 0.21, 95% CI = 0.062–0.85, p = 0.028); this implies better educational levels being protective. This is logical because a higher educational level is linked to improved blood glucose control, which is linked to good thyroid function [59].
Among the seven papers used to assess associated factors, two studies showed that previous family history of TD was associated with TD. A cross-sectional study conducted in Saudi Arabia among 411 T2DM patients found that diabetic patients with a positive family history of TD had a higher chance of developing TD (OR = 3.39, 95% CI: 2.47–4.63, p = < 0.0001) [23]. A study from Nepal also having previous family history of TD increased the risk by 2.57 (RR = 2.57, 95% CI = 2–3.31, p < 0.001) [32].
Age > 50 was significant factor with OR of 3.9 (95% CI 2.151–7.052, p < 0.001). This can be explained by that elderly patients might have had undetected diabetes for a longer time [44]. About the factor presence of retinopathy [59] with an odds ratio of 9.3 (95% CI: 2.05–42.51, p =0.04), and for factor presence of neuropathy [59] with an odds ratio of (OR =3.3, 95% CI =1.19–8.92, p =0.021) [32] showed the presence of retinopathy and neuropathy were risk factors of TD among T2DM patients respectively [39].
Conclusion and recommendation
The current systematic review and meta-analysis showed that the pooled prevalence of TD among T2DM patients was found to be higher compared with the general population. The pooled prevalence of TD among T2DM was found to be 20.24% (95% CI: 17.85, 22.64) using random-effect model. The pooled prevalence of subclinical hypothyroidism, hypothyroidism, subclinical hyperthyroidism, and hyperthyroidism was found to be 11.87% (95% CI: 6.90, 16.84), 7.75% (95% CI: 5.71, 9.79), 2.49% (95% CI: 0.73, 4.25), and 2.51% (95% CI: 1.89, 3.13), respectively. Being female, obesity, family history of TD, smoking, advanced age, and family history of DM were factors associated with TD among adult T2DM patients.
We recommend it is important to screen for TD in T2DM patients as each of these endocrinopathies and their complex interdependent interactions increase cardiovascular risks.
Strength and limitations
This systematic review and meta-analysis revealed the pooled figure on prevalence of TD, its subtypes, and associated factors of TD among T2DM patients. This will give researchers, policymakers, and public health stakeholders the empirical knowledge they need to develop health-promoting policies, allocate resources, and set priorities for monitoring future trends.
The limitations of this systematic review and meta-analysis is that the search strategy was limited only to published articles, but unpublished papers may be missed. Only free online databases were used. In addition to this, only papers written in English were included. Time barrier was also one of the limitations. Moreover, it is essential to highlight that the paucity of research conducted in the area of thyroid dysfunction among type 2 diabetes patients in Europe, America, and Australia resulted in a restricted number of articles being incorporated into our analysis.
Availability of data and materials
The main part of the data generated or analyzed during this study is included in this published article. Other data will be available from the corresponding author upon request.
Abbreviations
- BMI:
-
Body Mass Index
- DM:
-
Diabetes Mellitus
- FT3:
-
Free Triiodothyronine
- FT4:
-
Free Thyroxine
- HbA1C:
-
Hemoglobin A1C
- IDF:
-
International Diabetes Federation
- mIU/L:
-
Milli-international Unit per Liter
- NHANES:
-
National Health and Nutrition Examination Survey
- pmol/L:
-
Picomoles per Liter
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- TD:
-
Thyroid Dysfunction
- T2DM:
-
Type 2 Diabetes Mellitus
- TH:
-
Thyroid Hormone
- TRH:
-
Thyrotropin-releasing Hormone
- TSH:
-
Thyroid-stimulating Hormone
References
Wang C. The Relationship between type 2 diabetes mellitus and related thyroid diseases. J Diabetes Res. 2013;2013:390534.
Laulund AS, Nybo M, Brix TH, Abrahamsen B, Jørgensen HL, Hegedüs L. Duration of thyroid dysfunction correlates with all-cause mortality The OPENTHYRO Register Cohort. PloS one. 2014;9(10):e110437.
Ahmed AA, Mohamed SB, Elmadi SA, Abdorabo AA, Ismail IM, Ismail AM. Assessment of thyroid dysfunctions in type 2 diabetes mellitus patients in Surman, Western-Libya. Int J Clin Exp Med Sci. 2017;3:1–4.
DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1(1):1–22.
Inzucchi SE, Sherwin RS. Type 2 diabetes mellitus. Philadelphia, Pa: Saunders Elsevier; 2011.
Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27(4):269.
Diez JJ, Sánchez P, Iglesias P. Prevalence of thyroid dysfunction in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119(04):201–7.
Papanna J, Bettegowda S. Thyroid dysfunction among type 2 diabetes mellitus patients: a study from rural hospital. J Med Sci Clin Res. 2019;7:433–6.
Vikhe VB, Kanitkar SA, Tamakuwala KK, Gaikwad AN, Kalyan M, Agarwal RR. Thyroid dysfunction in patients with type 2 diabetes mellitus at tertiary care centre. Natl J Med Res. 2013;3(4):377–80.
Tudor RM, Garrahy A, Woods CP, Crowley RK, Tormey WT, Smith D, et al. The prevalence and incidence of thyroid dysfunction in patients with diabetes - a longitudinal follow-up study. Ir J Med Sci. 2020;189(1):171–5.
Subekti I, Pramono LA, Dewiasty E, Harbuwono DS. Thyroid dysfunction in type 2 diabetes mellitus patients. Acta Med Indones. 2017;49(4):314–23.
Kalra S, Aggarwal S, Khandelwal D. Thyroid dysfunction and type 2 diabetes mellitus: screening strategies and implications for management. Diabetes Ther. 2019;10(6):2035–44.
Datchinamoorthi S, Rathanavel N, Rajagopalan B, Vanaja R. Study of thyroid dysfunction in type II diabetes mellitus. Int J Pharm Sci Res. 2016;7(9):3877.
Moslem F, Bithi TS, Biswas A. Prevalence of thyroid dysfunction among type-2 diabetes patients in an urban diabetes hospital. Bangladesh Open Sci J Clin Med. 2015;3(3):98–113.
Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocr Rev. 2019;40(3):789–824.
Vamshidhar IS, Rani SSS. A study of association of thyroid dysfunctions in patients with type 2 diabetes mellitus. Maedica. 2020;15(2):169–73.
Hage M, Zantout MS, Azar ST. Thyroid Disorders and Diabetes Mellitus. J Thyroid Res. 2011;2011:439463, 7:1–7.
Ward RJ, Heald AH, Ogunmekan S, Fryer AA, Duff CJ. Should we be screening for thyroid dysfunction in patients with type 2 diabetes mellitus? Br J Gen Pract. 2018;68(667):94–5.
Mohammed Hussein SM, AbdElmageed RM. The relationship between type 2 diabetes mellitus and related thyroid diseases. Cureus. 2021;13(12):e20697.
Díez JJ, Iglesias P. Subclinical hyperthyroidism in patients with type 2 diabetes. Endocrine. 2012;42(1):157–63.
Vondra K, Vrbikova J, Dvorakova K. Thyroid gland diseases in adult patients with diabetes mellitus. Minerva Endocrinol. 2005;30(4):217–36.
Palma CC, Pavesi M, Nogueira VG, Clemente EL, Vasconcellos Mde F, Pereira LCJ, et al. Prevalence of thyroid dysfunction in patients with diabetes mellitus. Diabetol Metab Syndr. 2013;5(1):58.
Al-Geffari M, Ahmad NA, Al-Sharqawi AH, Youssef AM, AlNaqeb D, Al-Rubeaan K. Risk factors for thyroid dysfunction among type 2 diabetic patients in a highly diabetes mellitus prevalent society. Int J Endocrinol. 2013;2013:417920.
Akbar D, Ahmed M, Al-Mughales J. Thyroid dysfunction and thyroid autoimmunity in Saudi type 2 diabetics. Acta Diabetol. 2006;43(1):14–8.
Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. S Afr Fam Pract. 2012;54(5):384–90.
Reid JR, Wheeler SF. Hyperthyroidism: diagnosis and treatment. Am Fam Physician. 2005;72(4):623–30.
World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647.
Zhu Y, Xu F, Shen J, Liu Y, Bi C, Liu J, et al. Prevalence of thyroid dysfunction in older Chinese patients with type 2 diabetes-a multicenter cross-sectional observational study across China. PLoS ONE. 2019;14(5):e0216151.
Papazafiropoulou A, Sotiropoulos A, Kokolaki A, Kardara M, Stamataki P, Pappas S. Prevalence of thyroid dysfunction among Greek type 2 diabetic patients attending an outpatient clinic. J Clin Med Res. 2010;2(2):75.
Ogbonna SU, Ezeani IU. Risk factors of thyroid dysfunction in patients with type 2 diabetes mellitus. Front Endocrinol. 2019;10:440.
Khatiwada S, Kc R, Sah SK, Khan SA, Chaudhari RK, Baral N, et al. Thyroid dysfunction and associated risk factors among Nepalese diabetes mellitus patients. Int J Endocrinol. 2015;2015:570198.
Radaideh A, Mo M, Amari FL, Bateiha AE, El-Khateeb M, Naser P, et al. Diabetes mellitus in Jordan. Saudi Med J. 2004;25(8):1046–50.
Ozair M, Noor S, Raghav A, Siddiqi SS, Chugtai AM, Ahmad J. Prevalence of thyroid disorders in North Indian type 2 diabetic subjects: a cross sectional study. Diabetes Metab Syndr. 2018;12(3):301–4.
Elgazar EH, Esheba NE, Shalaby SA, Mohamed WF. Thyroid dysfunction prevalence and relation to glycemic control in patients with type 2 diabetes mellitus. Diabetes Metab Syndr. 2019;13(4):2513–7.
Younes YR. The prevalence of thyroid dysfunction in patients with type 2 diabetes mellitus. Chronic Dis J. 2020;8(1):45–8.
Haque SIU, Syed TM, Tahir A. Burden of thyroid dysfunction in patients of type 2 diabetes mellitus. PAFMJ. 2019;69(4):843–7.
Aljabri KS, Alnasser IM, Facharatz BS, Bokhari SA, Alshareef MA, Khan PM. The frequency of hypothyroidism in Saudi community-based hospital: A retrospective single centre study. Trends Diabetes Metab. 2019;2(1):1–4.
Al-Wazzan H, Daban A, Askar R, El-Shazly M. Prevalence and associated factors of thyroid dysfunction among type 2 diabetic patients. Kuwait Alexandria Journal of Medicine. 2010;46(2):141–8.
Jain G, Marwaha TS, Khurana A, Dhoat PS. Prevalence of thyroid disorders in patients of type 2 diabetes mellitus. Int J Med Dent Sci. 2013;2(2):153–61.
Nair A, Jayakumari C, Jabbar PK, Jayakumar RV, Raizada N, Gopi A, et al. Prevalence and associations of hypothyroidism in Indian patients with type 2 diabetes mellitus. J Thyroid Res. 2018;2018:5386129.
Jalal MJA, Riyas B, Kumar AP. Thyroid dysfunction in patients with type-2 diabetes mellitus in Kerala: a case–control study. Thyroid Res Pract. 2019;16(1):3.
Ghazali S, Abbiyesuku F. Thyroid dysfunction in type 2 diabetics seen at the University College Hospital, Ibadan. Nigeria Niger J Physiol Sci. 2010;25(2):173–9--9.
Khassawneh AH, Al-Mistarehi AH, Zein Alaabdin AM, Khasawneh L, AlQuran TM, Kheirallah KA, et al. Prevalence and predictors of thyroid dysfunction among type 2 diabetic patients: a case-control study. Int J Gen Med. 2020;13:803–16.
Sarfo-Kantanka O, Sarfo FS, Ansah EO, Yorke E, Akpalu J, Nkum BC, et al. Frequency and determinants of thyroid autoimmunity in Ghanaian type 2 diabetes patients: a case-control study. BMC Endocr Disord. 2017;17(1):2.
Padma V, Anand NN. Prevalence of Thyroid Dysfunction in Type 2 Diabetic Patients. Int J Pharm Biochem Sci. 2015;6(3):289–94.
Hussain T, Barik BS, Nayak AR, Das S, Khadanga UK, Yadav V, et al. Prevalence and predictors of thyroid dysfunction among patients with type 2 diabetes mellitus attending a tertiary care hospital in an urban area of Bhubaneswar, Odisha. Thyroid Res Pract. 2019;16(1):26.
Peters KE, Chubb SAP, Bruce DG, Davis WA, Davis TME. Prevalence and incidence of thyroid dysfunction in type 1 diabetes, type 2 diabetes and latent autoimmune diabetes of adults: the Fremantle Diabetes Study Phase II. Clin Endocrinol. 2020;92(4):373–82.
Yadav A, Yadav GAM, Narsingrao KK, Nanda Kumar LG, Yadav GSN. Prevalence of thyroid disorders among patients with diabetes in rural South India. Diabetes Metab Syndr. 2021;15(3):885–9.
Demitrost L, Ranabir S. Thyroid dysfunction in type 2 diabetes mellitus: a retrospective study. Indian J Endocrinol Metab. 2012;16(Suppl 2):S334.
Elmenshawi I, Alotaibi S, Alazmi A, Alazmi A, Alruwaili F, Alazmi N, et al. Prevalence of thyroid dysfunction in diabetic patients. J Diabetes Metab Disord. 2017;4:55–6.
Al-Lehibi KI, Abdulrahman MI, Albassam ENA-A. Thyroid dysfunction in type 2 diabetic patients and the effect of diabetes duration and anti-glycemic medications on mean tsh and a1c levels: a retrospective study. Int J Med Res Health Sci. 2019;8(9):117–22.
Jugati A, Biradar M. Thyroid dysfunction in patients with type 2 diabetes mellitus in a tertiary care center of North Karnataka. Medica. 2018;7(2):28.
Aljabri KS. The prevalence of thyroid disorders in patients with type 2 diabetes mellitus in Saudi community based hospital. Curr Res Diabetes Obes J. 2019;11(3):60–4.
Vij V, Chitnis P, Gupta VK. Evaluation of thyroid dysfunction among type II diabetic patients. Ijpbs. 2012;2(4):150–5.
Jali M, Kambar S, Jali SM, Pawar N, Nalawade P. Prevalence of thyroid dysfunction among type 2 diabetes mellitus patients. Diabetes Metab Syndr. 2017;11:S105–8.
Zeru MA, Tesfa E, Mitiku AA, Seyoum A, Bokoro TA. Prevalence and risk factors of type-2 diabetes mellitus in Ethiopia: systematic review and meta-analysis. Sci Rep. 2021;11(1):1–15.
Zafar M, Shahid SM, Alshammari RF, Kausar MA, Ginawi TA, Hatim AW, Wadi AM, Ali H, Hamed AA, Al-zahrani MS, Hussain A. Association of thyroid disorders with diabetes: A cross-sectional study. Nus Biosci. 2022;14(2).
Tekalign AM, Habte FB, Yimer RM. Determinants of Thyroid Dysfunction among Type 2 Diabetes Patients Attending Private Hospitals in Dire Dawa, Eastern Ethiopia. medRxiv. 2022:2022–02.
Grassetto G, Rubello D. Thyroid disorders and diabetes mellitus. Minerva Med. 2008;99(3):263–7.
Han C, He X, Xia X, Li Y, Shi X, Shan Z, et al. Subclinical hypothyroidism and type 2 diabetes: a systematic review and meta-analysis. PLoS ONE. 2015;10(8):e0135233.
Moini J, Pereira K, Samsam M. Epidemiology of thyroid disorders. Elsevier; 2020 Jan 8. 75-85
Joffe BI, Distiller LA. Diabetes mellitus and hypothyroidism: strange bedfellows or mutual companions? World J Diabetes. 2014;5(6):901.
Chiovato L, Magri F, Carlé A. Hypothyroidism in context: where we’ve been and where we’re going. Adv Ther. 2019;36(2):47–58.
de Benoist B, Andersson M, Takkouche B, Egli I. Prevalence of iodine deficiency worldwide. The Lancet. 2003;362(9398):1859–60.
Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol. 1977;7(6):481–93.
Alam Khan V, Khan MA, Akhtar S. Thyroid disorders, etiology and prevalence. J Med Sci. 2002;2(2):89–94.
Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14(5):301–16.
Ogbera AO, Kuku SF. Epidemiology of thyroid diseases in Africa. Indian journal of endocrinology and metabolism. 2011;15(Suppl2):S82.
Okosieme OE. Impact of iodination on thyroid pathology in Africa. J R Soc Med. 2006;99(8):396–401.
Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10(8):594–604.
Jacobson MH, Howards PP, Darrow LA, Meadows JW, Kesner JS, Spencer JB, et al. Thyroid hormones and menstrual cycle function in a longitudinal cohort of premenopausal women. Paediatr Perinat Epidemiol. 2018;32(3):225–34.
Babić Leko M, Gunjača I, Pleić N, Zemunik T. Environmental factors affecting thyroid-stimulating hormone and thyroid hormone levels. Int J Mol Sci. 2021;22(12):6521.
Author information
Authors and Affiliations
Contributions
Rishan Hadgu conducted study design, conception of research protocol, literature review, and data extraction. Sintayehu Ambachew, Abebaw Worede, and Rishan Hadgu were involved in data analysis and interpretation and manuscript drafting. Abebaw Worede and Sintayehu Ambachew made data interpretation and reviewed the manuscript. Rishan Hadgu and Sintayehu Ambachew were responsible for data extraction and quality assessment. All the authors critically revised the paper and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants provided written informed consent to publish this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hadgu, R., Worede, A. & Ambachew, S. Prevalence of thyroid dysfunction and associated factors among adult type 2 diabetes mellitus patients, 2000–2022: a systematic review and meta-analysis. Syst Rev 13, 119 (2024). https://doi.org/10.1186/s13643-024-02527-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-024-02527-y