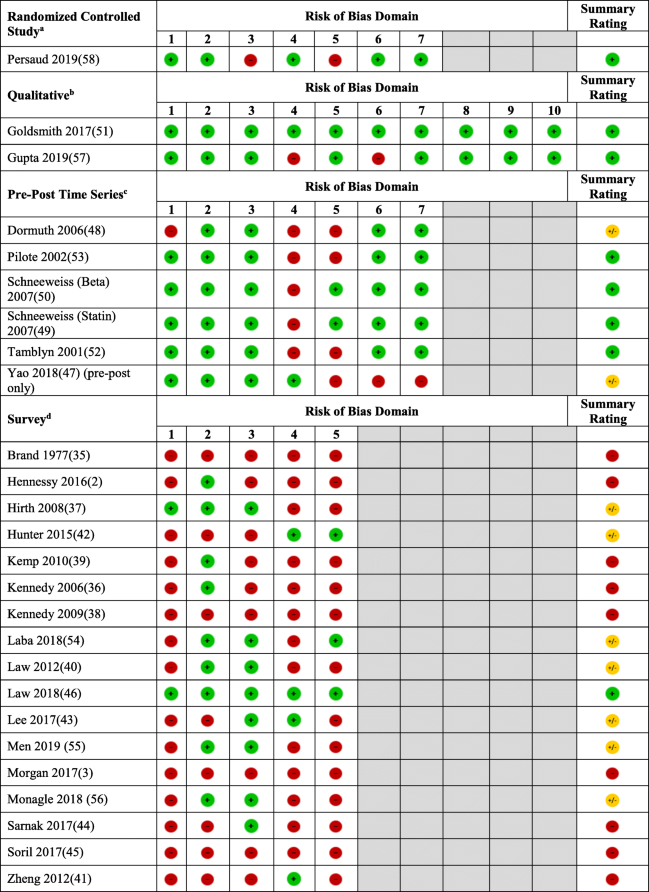
- aRoB domains for randomized controlled studies (1 = random sequence generation; 2 = allocation concealment; 3 = blinded participants and providers; 4 = blinded outcome assessors; 5 = incomplete outcome data; 6 = selective reporting; 7 = other biases)
- bRoB domains for qualitative studies (1 = clear statement of aims; 2 = qualitative methods justified; 3 = appropriate design for research aims; 4 = appropriate recruitment strategy; 5 = confidence in data collection; 6 = personal biases; 7 = ethical considerations; 8 = confidence in data analysis; 9 = clear statement of findings; 10 = value of research)
- cRoB domains for pre-post time series studies (1 = confounding variables/events; 2 = analysis at point of intervention; 3 = intervention effects on data collection; 4 = blinding or objective outcomes; 5 = effect of missing outcome measures; 6 = selective reporting; 7 = other biases)
- dRoB domains for surveys (1 = representativeness of sample; 2 = adequacy of response rate; 3 = missing data; 4 = pilot testing; 5 = published validity of survey instrument).
 denotes a high risk of bias,
denotes a high risk of bias,  denotes a moderate risk of bias, and
denotes a moderate risk of bias, and  denotes a low risk of bias)
denotes a low risk of bias)
